Page 53 - 新思维科学学生用书7 样章
P. 53
2 Materials and their structure
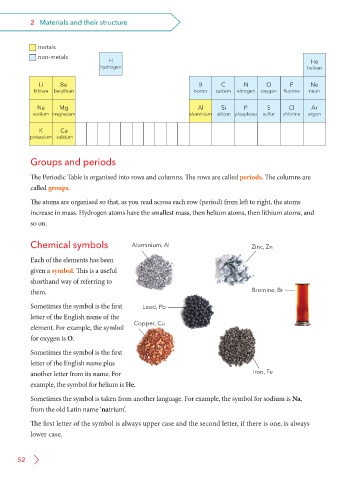
metals
non-metals H He
hydrogen helium
Li Be B C N O F Ne
lithium beryllium boron carbon nitrogen oxygen fluorine neon
Na Mg Al Si P S Cl Ar
sodium magnesium aluminium silicon phosphorus sulfur chlorine argon
K Ca
potassium calcium
Groups and periods
The Periodic Table is organised into rows and columns. The rows are called periods. The columns are
called groups.
The atoms are organised so that, as you read across each row (period) from left to right, the atoms
increase in mass. Hydrogen atoms have the smallest mass, then helium atoms, then lithium atoms, and
so on.
Chemical symbols Aluminium, Al Zinc, Zn
Each of the elements has been
given a symbol. This is a useful
shorthand way of referring to
them. Bromine, Br
Bromine, Br
Sometimes the symbol is the first Lead, Pb
letter of the English name of the
element. For example, the symbol Copper, Cu
for oxygen is O.
Sometimes the symbol is the first
letter of the English name plus
another letter from its name. For Iron, Fe
example, the symbol for helium is He.
Sometimes the symbol is taken from another language. For example, the symbol for sodium is Na,
from the old Latin name ‘natrium’.
The first letter of the symbol is always upper case and the second letter, if there is one, is always
lower case.
52