Page 60 - 新思维科学学生用书7 样章
P. 60
2.6 Compounds and formulae
Using formulae
Every compound has a chemical name. For example, the compound of sodium and chlorine is sodium
chloride. Some compounds also have an everyday name. For example, sodium chloride is also known
as common salt.
Every compound also has a formula (the plural of this word is formulae). The formula contains the
symbols of the elements that are bonded together in the compound.
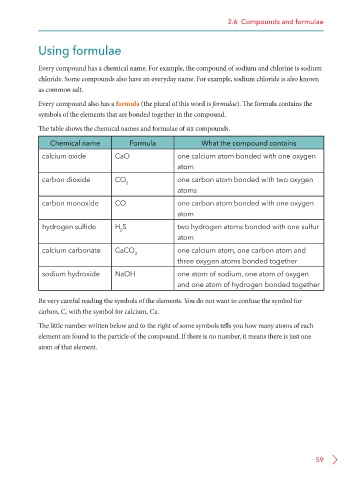
The table shows the chemical names and formulae of six compounds.
Chemical name Formula What the compound contains
calcium oxide CaO one calcium atom bonded with one oxygen
atom
carbon dioxide CO 2 one carbon atom bonded with two oxygen
atoms
carbon monoxide CO one carbon atom bonded with one oxygen
atom
hydrogen sulfide H S two hydrogen atoms bonded with one sulfur
2
atom
calcium carbonate CaCO 3 one calcium atom, one carbon atom and
three oxygen atoms bonded together
sodium hydroxide NaOH one atom of sodium, one atom of oxygen
and one atom of hydrogen bonded together
Be very careful reading the symbols of the elements. You do not want to confuse the symbol for
carbon, C, with the symbol for calcium, Ca.
The little number written below and to the right of some symbols tells you how many atoms of each
element are found in the particle of the compound. If there is no number, it means there is just one
atom of that element.
59