Page 66 - 新思维科学学生用书7 样章
P. 66
2.7 Compounds and mixtures
Continued
Questions
1 Describe the appearance of:
a a mixture of iron and sulfur
b the iron sulfide.
2 Can you remove the iron from the iron sulfide by using a magnet? Explain your
answer.
Air is a mixture
When you mix iron and sulfur together, you make a mixture 78% nitrogen
of two elements.
In science, the word pure is used to describe something that
only contains a single substance. Pure water contains only
water, with no other substances mixed with it.
A mixture is not pure. It is made up of different kinds of
particle that are mixed together. The mixture may be of
elements, compounds or both. There are solids, liquids and 1% carbon dioxide, 21% oxygen
gases that are mixtures. argon, water vapour
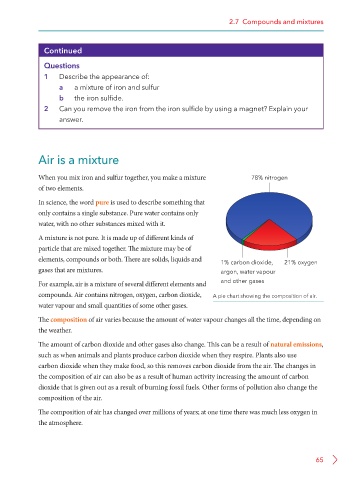
For example, air is a mixture of several different elements and and other gases
compounds. Air contains nitrogen, oxygen, carbon dioxide, A pie chart showing the composition of air.
water vapour and small quantities of some other gases.
The composition of air varies because the amount of water vapour changes all the time, depending on
the weather.
The amount of carbon dioxide and other gases also change. This can be a result of natural emissions,
such as when animals and plants produce carbon dioxide when they respire. Plants also use
carbon dioxide when they make food, so this removes carbon dioxide from the air. The changes in
the composition of air can also be as a result of human activity increasing the amount of carbon
dioxide that is given out as a result of burning fossil fuels. Other forms of pollution also change the
composition of the air.
The composition of air has changed over millions of years; at one time there was much less oxygen in
the atmosphere.
65