Page 30 - 新思维科学学生用书7 样章
P. 30
2.1 Solids, liquids and gases
Particle theory
All matter is made up of tiny particles that are much too small to see. The particles are arranged
differently in solids, liquids and gases.
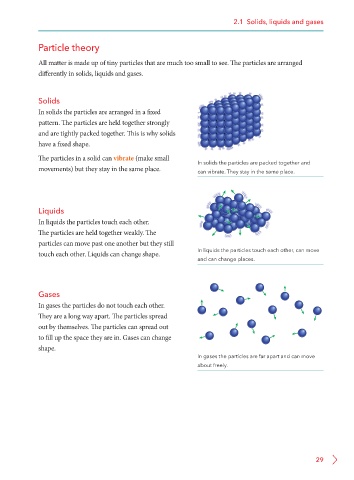
Solids
In solids the particles are arranged in a fixed
pattern. The particles are held together strongly
and are tightly packed together. This is why solids
have a fixed shape.
The particles in a solid can vibrate (make small In solids the particles are packed together and
movements) but they stay in the same place. can vibrate. They stay in the same place.
Liquids
In liquids the particles touch each other.
The particles are held together weakly. The
particles can move past one another but they still
touch each other. Liquids can change shape. In liquids the particles touch each other, can move
and can change places.
Gases
In gases the particles do not touch each other.
They are a long way apart. The particles spread
out by themselves. The particles can spread out
to fill up the space they are in. Gases can change
shape.
In gases the particles are far apart and can move
about freely.
29