Page 32 - 新思维科学学生用书7 样章
P. 32
2.1 Solids, liquids and gases
Explaining the properties
Matter can only flow (be poured) if the particles can
move past one another.
Matter can only change volume if the particles in it can
spread out or move closer together.
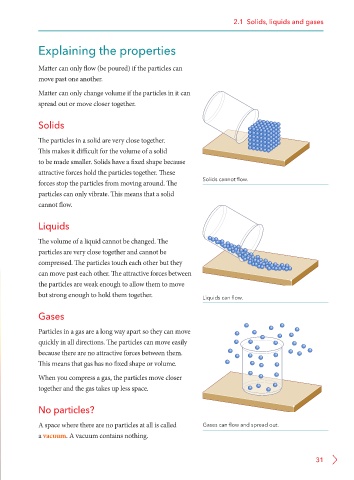
Solids
The particles in a solid are very close together.
This makes it difficult for the volume of a solid
to be made smaller. Solids have a fixed shape because
attractive forces hold the particles together. These
forces stop the particles from moving around. The Solids cannot flow.
particles can only vibrate. This means that a solid
cannot flow.
Liquids
The volume of a liquid cannot be changed. The
particles are very close together and cannot be
compressed. The particles touch each other but they
can move past each other. The attractive forces between
the particles are weak enough to allow them to move
but strong enough to hold them together. Liquids can flow.
Gases
Particles in a gas are a long way apart so they can move
quickly in all directions. The particles can move easily
because there are no attractive forces between them.
This means that gas has no fixed shape or volume.
When you compress a gas, the particles move closer
together and the gas takes up less space.
No particles?
A space where there are no particles at all is called Gases can flow and spread out.
a vacuum. A vacuum contains nothing.
31