Page 41 - 新思维科学学生用书7 样章
P. 41
2 Materials and their structure
Changes of state
Heating solids
When solids are heated they expand (get bigger).
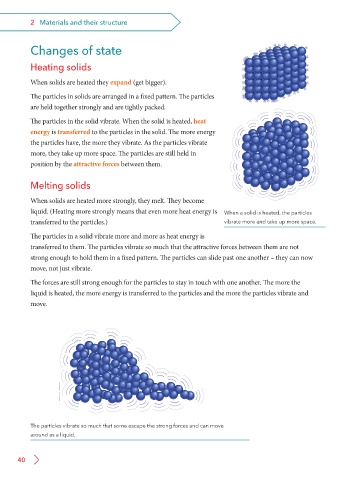
The particles in solids are arranged in a fixed pattern. The particles
are held together strongly and are tightly packed.
The particles in the solid vibrate. When the solid is heated, heat
energy is transferred to the particles in the solid. The more energy
the particles have, the more they vibrate. As the particles vibrate
more, they take up more space. The particles are still held in
position by the attractive forces between them.
Melting solids
When solids are heated more strongly, they melt. They become
liquid. (Heating more strongly means that even more heat energy is When a solid is heated, the particles
transferred to the particles.) vibrate more and take up more space.
The particles in a solid vibrate more and more as heat energy is
transferred to them. The particles vibrate so much that the attractive forces between them are not
strong enough to hold them in a fixed pattern. The particles can slide past one another – they can now
move, not just vibrate.
The forces are still strong enough for the particles to stay in touch with one another. The more the
liquid is heated, the more energy is transferred to the particles and the more the particles vibrate and
move.
The particles vibrate so much that some escape the strong forces and can move
around as a liquid.
40